A blog by Robert Ferris, Ph.D.
Regulation of Nanotechnology
The nanotechnology regulatory environment is fraught with convolution, controversy, and confusion; here is why.
As it stands today, the production of engineered nanotechnology-based products are not subject to special regulation regarding manufacturing, handling, or testing. No special labels are required on products. No special personal protection equipment is required to be worn my manufacturing personal. And no tests are enforced to assess how a nano-based product interacts with people or the environment.
In large part, this is because most engineered nanomaterials are not known to cause biological or environmental issues. Yes, we there are known issues with inhalation of carbon nanotubes or potassium titanium oxide; but these issues are exceptions to most of what is being manufactured today. Nanotechnology is not new; humans and nature have been operating on the nano-length scale since before we coined the term nanotechnology. The only thing that has changed in the past 20 years is our level of sophistication around manipulating materials at the nano-length scale. In part, the need for regulation is due to production of non-natural engineering nanomaterials and the unknown effects once introduced into the body or environment.
So, what is the real risk with the unknowns of nanotechnology? Believe it or not, the real risk is not a sudden release of a material that induces immediate human death or environmental destruction. The real risk is a systemic or chronic accumulation of a nanomaterial that induces irreversible damage only after prolonged exposure or a long delay; something like a carcinogen such as asbestos. Testing for chronic exposure limits is extremely difficult and nearly impossible with a field as vast as nanotechnology. For example, carbon nanotubes were discovered in 2006 but only recently were exposure limits set limit their adverse effects on lungs. The problem with carcinogenic material is that it only presents symptoms long-after exposure. By then, a large amount of material could have entered the environment.
Many production companies have produced nanomaterials for some time now, complicating any new broad-sweeping regulatory changes. The recent explosion of new compounds, ranging from nanoparticles to nanocomposites, has strained regulatory resources and capabilities in grasping this problem. Even today fundamental questions about how nanomaterials interact with complex environments remain unanswered. Regulatory agencies are unsure how to handle the new engineering materials entering the market. In a recent quote by Kristen Kulinowski (ICON): “We are in this awkward middle territory where we have just enough information to think there is an issue, but not enough information to really inform policymakers about what to do about it.” This information lag between products being introduced to the market and the analysis by regulatory agencies is reflected in Figure X1, presented by Gregory Lowry who is part of the Center for Environmental Implications of Nanotechnolgoy (CEINT) at Carnegie Mellon University. Market innovation will always forge ahead and leave government regulation to try and keep up. Regardless, many groups are calling for stricter regulation of nanomaterials; ranging from scientists, innovators, environmentalists and general public interest. This has driven nearly every regulatory agency in nearly every government around the world to enact some form of a nanotechnology task force.
Right now, a number of international regulatory agencies are working on the problem. In the United States government, we have the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), the Center of Disease Control (CDC), and the National Institute for Standards and Technology (NIST). Within each of these there are countless organizations serving providing insight, studies, and guidance. The EPA seeks to control nanoscale materials through the Toxic Substance Control Act and the Federal Insecticide, Fungicide, and Rodenticide Act. The FDA organized a task force in 2006 to determine a regulatory approaches that encourage the continued development of innovative, safe, and effective FDA-regulated products that use nanotechnology materials (their last formal report was in 2007). The CDC uses the National Institute of Occupational Safety and Health (NIOSH) to assess 10 critical topic areas regarding nanotechnology, including; toxicity and dose, surveillance, PPE, fire & explosion safety, and measurement methods. NIST operates the Center for Nanoscale Science and Technology (CNST), that provides public and private center agencies with access to world-class nanoscale measurement and fabrication technology. If you aren’t confused yet, feel free to research the European agency Health and Consumer Protection Directorate (HCPD) or the Chinese National Steering Committee for Nanoscience and Nanotechnology (NSCNN).
This ad hoc government engagement means that there is no homogeneous message or voice of authority regarding the manufacturing, handling, or tracking of nanoscale materials. In fact, a number of these organizations have been working for over 10 years and still have not produced basice toxicity testing protocols. A recent report by the Woodrow Wilson Centre’s Project on Emerging Technologies conclude that there is still a limited understanding of the human health and safety risks associated with nanotechnology. The EPA is approaching the issue from the standpoint of the nanomaterial life cycle assessments; where they try to assess how nanomaterials interact with the environment from cradle to grave. To start this effort they are requesting nanomaterial manufacturers to submit a Significant New Use Notice (SNUN) to the EPA at least 90 days before commencing manufacturing. As each agency issues similar requests, companies find themselves juggling potentially crippling government studies and assessments, which leaves a lot of uncertainty around what manufacturing techniques constitute “nano” or who’s rules need to be followed.
A lot of this uncertainty revolves around the unknowns about nanomaterials. The truth is that we still do not know how these materials interact with the environment or humans. Each material is different. Even a shape change can change the material properties (e.g. from a sphere to a wire). Figure X2 presents 6 aspects that can influence nanomaterial properties. Compound each of these aspects across countless compositions and you see the problem government agencies are facing. The agencies cannot keep up nor can they lock-down the rules without supporting data. This complicates the funding mess, because companies are forging ahead with product innovations and offerings.
This leaves many companies having to either develop their own testing techniques, or assume a great deal of financial risk in a new nanomaterial. In a recent article in the economist, Michael Holman of Lux Research concluded that, “larger companies can probably cope with the research because they are more familiar with the risks of liability and regulation. But the task is beyond some small companies.” (Link) The alternative of a complete nanomaterial lock down is equally detrimental to innovation, with many companies incurring regulatory costs through unnecessary, inspections, assessments, and oversight by multiple agencies. Because of this regulatory confusion, there is a strong need for clear governmental direction and investment in testing agencies.
The uncertainty in regulation has already impacted the rate of nanomaterial introduction into the market place. Some can think this is a well-justified inhibitor to innovation while others see this is a loss of potential gains. Regardless of the reason, the marketplace will benefit from each governmental agency to focus and streamline how they handle the nanotechnology revolution. In it’s current state the overlapping approaches actually leave gaps in regulation role-out that allows for potentially harmful nanomaterials to enter the market place even when a clear testing standard and regulation is in place.
Stay tuned: My next post will address the recent trials and tribulations of nanomanufacturing
Robert Ferris, Ph.D. is a strategic planner with Emerson Process Managements. He holds a bachelors and masters in chemical engineering, an MBA in new technology commercialization, and a Ph.D. in Mechanical Engineering and Materials Science. He has an extensive background in nanotechnology development and advanced process control.
Please, share you opinion or questions! Reply below to provide your thoughts, insights, or comments with the experts.
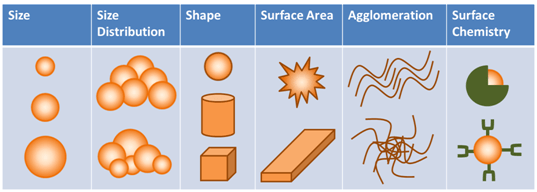
Figure 1: Nanomaterial characteristics that can affect life-cycle interactions (Source: GAO-14-181SP: Published: Jan 31, 2014)
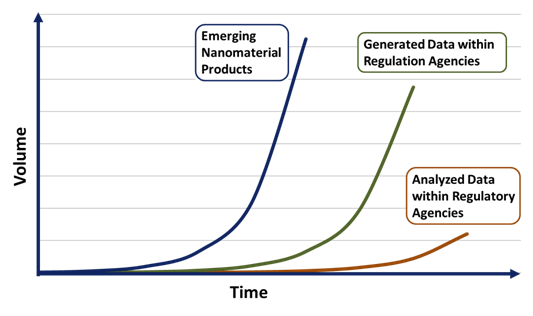
Figure 2: Schematic of the information lag between nanotechnology innovation and regulatory agencies.




